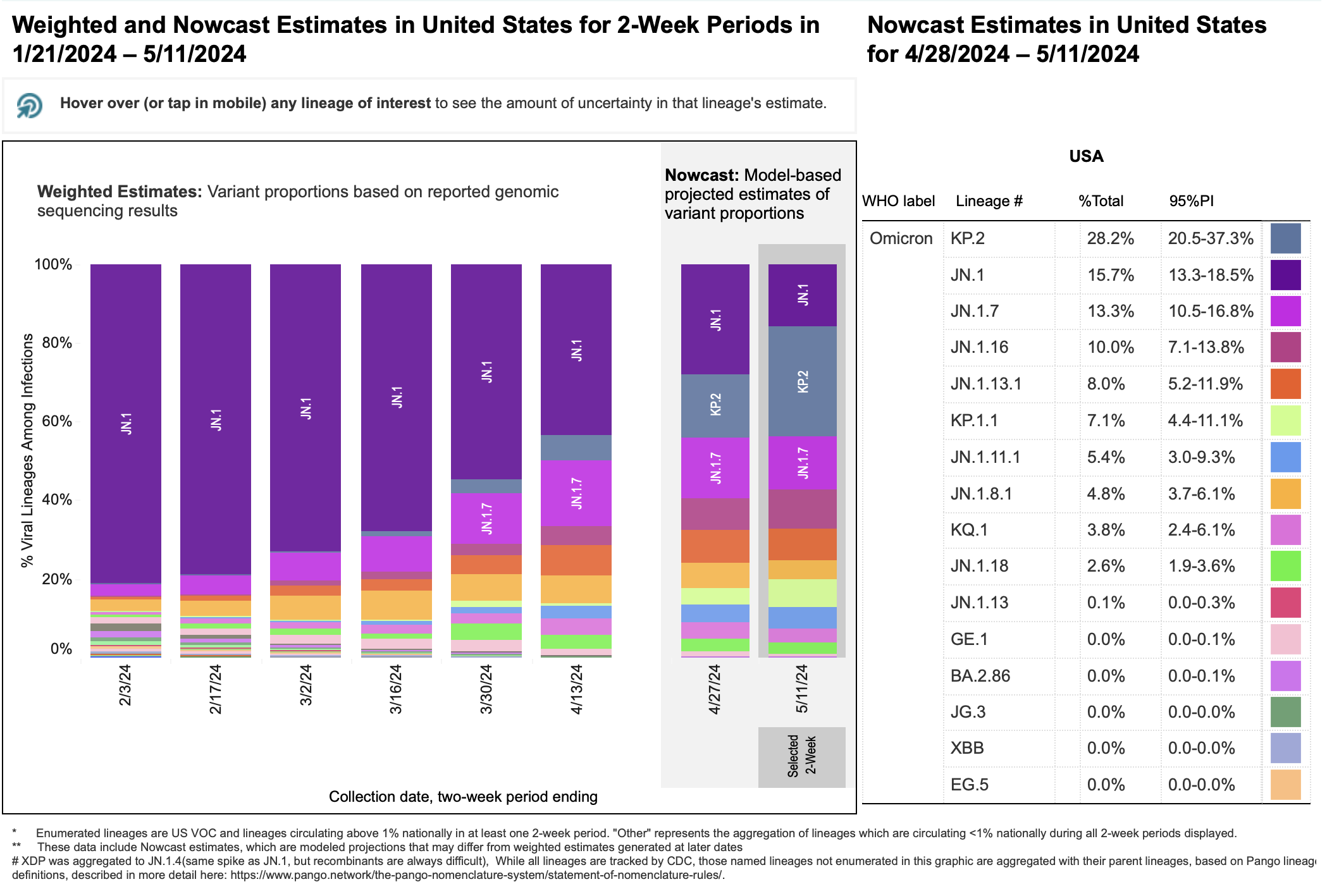
As of May 14, 2024, the SARS-CoV-2 Omicron variants JN.1 and a JN.1 descendant, KP.2, have high prevalence in the United States. CDC Nowcast projections estimate KP.2 (also called JN.1.11.1.2), to account for nearly 30% of new COVID-19 illnesses in the U.S. The proportion of illnesses caused by KP.2 is rapidly increasing, from 3.9% estimated during the week of March 30 to 28.2% estimated during the week of May 11, 2024.
Pictured above is a figure showing recent emergence of JN.1, KP.2, and other Omicron strains in the U.S. For additional infographics depicting the change in variant proportions in the U.S. over time and/or geographically or phylogenetically, visit the CDC’s COVID Data Tracker resource.
Immunity, Transmissibility and Vaccines: Last Updated May 14, 2024
Seroprevalence of SARS-CoV-2 and Omicron-specific antibodies
A very high proportion (>95%) of individuals currently have identifiable antibodies against SARS-CoV-2, either from infection or immunization or a combination of both. A recent large serosurvey aimed at investigating mucosal immunity in the Netherlands also identified very high (95%) spike-specific IgG in nasal samples of individuals.
Existing research in the U.S. indicates a large increase in SARS-CoV-2 antibody seroprevalence from the pre-Omicron to Omicron era, across all age groups; it was estimated that by the third quarter of 2022, approximately two-thirds of individuals aged 16 years and older had been infected with SARS-CoV-2, with approximately half of individuals having hybrid immunity.
JN.1-specific immunity and transmissibility
The JN.1 variant is a subvariant of Omicron variant BA.2.86, and contains several mutations that are associated with escape from vaccine-mediated immune protection. Recent research shows that JN.1 is very efficient at immune evasion (even more so than other Omicron variants), resulting in an increased reproductive number. Evidence from a small serological study has suggested that serological protection against SARS-CoV-2 is reduced against JN.1 variants compared to other BA.2.86 viruses, among young adults who had received at least a complete primary series of SARS-CoV-2 vaccines. Additionally, a recent serological survey of 1,472 community-dwelling individuals found that although a majority of previously-infected individuals had antibodies with neutralizing activity against JN.1, the neutralizing capacity was relatively low compared to neutralizing capacity against other SARS-CoV-2 strains. These findings are supported by an additional antigenic cartography analysis, which indicates that although XBB.1.5 booster sera was capable of neutralizing XBB sublineage variants (including JN.1), a five-fold titer difference was still observed.
Although JN.1 does appear to be more transmissible, it does not appear to cause more severe disease than other SARS-CoV-2 variants.
KP.2-specific immunity and transmissibility
The KP.2 variant (also called JN.1.11.1.2) is a descendant of the JN.1 variant and contains several mutations that are associated with escape from vaccine-mediated immune protection. Preliminary research (not yet peer-reviewed) suggests that the estimated relative effective reproduction number of KP.2 (Re) may be 1.22 times higher than the Re for JN.1.
This preliminary finding suggests higher viral fitness than other JN.1 variants and subvariants, although a pseudovirus assay in this research suggested that the infectivity of KP.2 may be 10.5-fold lower than JN.1. Importantly, in virus neutralization assays, KP.2 showed substantial resistance to sera of individuals vaccinated with monovalent XBB.1.5 (i.e., the most recently updated COVID-19 vaccine). However, because of the high antigenic similarity between KP.2 and JN.1, it is expected that individuals with a recent JN.1 infection will likely have some cross-neutralizing antibody protection against KP.2.
KP.2 is a member of a group of SARS-CoV-2 variants sometimes called “FLiRT” variants (so named because of the technical names for their mutations: F for L at position 456, and R for T at position 346). Other FLiRT variants, including KP.1.1, have also been identified as circulating in the US, but have not yet become as widespread as KP.2. (KP.1.1 is currently projected to account for approximately 7.5% of new COVID-19 illnesses in the US.)
Vaccines and current SARS-CoV-2 variants
Vaccination is still effective in preventing severe COVID-19, and vaccination with up-to-date SARS-CoV-2 vaccines does produce antibodies that can recognize JN.1.
Research has identified that JN.1 is resistant to monovalent XBB.1.5 vaccine sera, although data also suggest that JN.1 is unlikely to completely evade T cell recognition. A recent report has identified substantially higher neutralizing antibodies elicited by bivalent (ancestral strain plus BA.4/BA.5) vaccines against several Omicron subvariants, including BA.2.86 and JN.1, compared to the original monovalent vaccine. There is very limited evidence to estimate neutralizing capacity of current vaccine-elicited antibody against KP.2 strains. Due to the antigenic similarity between KP.2 and JN.1, it is likely that the neutralizing capacity against KP.2 would be similar to neutralizing capacity against JN.1.
Preliminary estimates of vaccine effectiveness (VE) against disease likely caused by JN.1 are imprecise due to the recent emergence of JN.1 in the United States and the subsequent rapid rise of KP.2 taking its place. Although those preliminary estimates suggest that there is still substantial VE against JN.1 (VE: 49%; 95% confidence interval: 19 – 68%), the VE estimate is lower than the estimate against non-JN.1 illnesses (VE: 60%; 95% confidence interval: 35 – 75%). Estimates for JN.1-specific vaccine effectiveness were lower in a large vaccine effectiveness study of Cleveland Clinic employees (19% VE). These U.S.-based estimates for XBB.1.5 vaccine effectiveness against JN.1 were similar to preliminary estimates from a recent pre-print using data from the Netherlands (41% VE in 18-59-year-olds, and 50% in 60-85 year-olds). However, estimates for JN.1-specific vaccine effectiveness were lower in a large vaccine effectiveness study of Cleveland Clinic employees (19% VE). There is very limited evidence to estimate effectiveness of current vaccines against KP.2. Given the antigenic similarity between JN.1 and KP.2, VE against KP.2 is likely to be similar to VE against JN.1, but may be lower.
Due to these combined factors, the Advisory Committee on Immunization Practices recommended in late February 2024 that people 65 years of age and older should get an additional updated COVID-19 vaccine in spring 2024.
Diagnostic Capacity for Variants: Last Updated May 14, 2024
Limited evidence available suggests that COVID-19 antigen and PCR tests are still capable of identifying recently-emerged SARS-CoV-2 variants, such as XBB.1.5, EG.5.1, JN.1 and KP.2. Reduction or failure of the spike gene amplification in RT-PCR can be used as a (time-dependent) proxy indicator of JN.1 (versus other Omicron lineages) infection.
Therapeutics: Last Updated May 14, 2024
Paxlovid continues to be effective against emerging SARS-CoV-2 variants, including JN.1 and KP.2.